Enhancing Operational Stability of TADF OLEDs
Key Points
- Researchers from Tsinghua University investigated the effects of targeted deuteration on donor and/or acceptor units of TADF emitters to enhance device operational stability, using the Edinburgh Instruments FS5 Spectrofluorometer, the FLS Photoluminescence Spectrometer and the LP980 Transient Absorption Spectrometer.
- Enhancing blue OLEDs’ operational stability remains an ongoing challenge for the photonics and semiconductor industry.
- Molecular engineering strategies at the subatomic level are promising methods for developing efficient blue OLED-based devices.
- The findings were recently published in Nature Communications.
Introduction
Developing efficient blue organic light-emitting diodes (OLEDs) is a critical challenge in OLED research and industry. One promising mechanism to boost the efficiency of OLEDs is thermally activated delayed fluorescence (TADF), which improves their photophysical properties by harvesting triplet excitons.
This Research Highlight summarises the findings of our spectrometer users at Tsinghua University in China, who have recently published their innovative work in Nature Communications. Their focus on the molecular engineering of chemical structures to enhance the efficiency of TADF compounds represents a significant advancement in our understanding of OLED technology.
Specifically, the researchers explored the kinetic isotopic effect (KIE), which occurs when a material’s atom is replaced by its isotope, leading to a change in the rate of the chemical reaction. The most common isotopic effect occurs with deuteration, which is the replacement of a proton (or protium) by deuterium. The substitution with a heavier isotope can enhance the molecule’s stability as the activation energy for bond dissociation is increased. However, the use of deuteration to improve molecular stability in OLED applications has been relatively unexplored.
The authors focused on the independent deuteration of donor and acceptor units, studying the KIE in oxygen-bridged triarylboron units with targeted deuteration of diphenylamine (DPA) and/or phenoxy groups on the 5,9-dioxa-13b-bor-anaphtho[3,2,1-de]anthracene (BO) moieties, namely DPA-BO, d-DPA-BO, DPA-D-BO and d-DPA-d-BO. The results of their study suggest that by increasing the degree of deuteration, device operational stability and prolonged lifetime of TADF-based OLEDs can be achieved, marking a significant advancement in the field.
Experimental Setup
The photophysics of the deuterated TADF compounds was studied using an Edinburgh Instruments FS5 Spectrofluorometer, an FLS Photoluminescence Spectrometer and an LP980 Transient Absorption Spectrometer (Figures 1a, 1b, and 1c, respectively). Photoluminescence (PL) spectra of the compounds were acquired using the FS5, while the FLS spectrometer with an EPL-375 picosecond pulsed laser diode and TCSPC electronics were used to measure the PL decays. Time-gated spectra of the prompt and delayed emission were measured using the iCCD camera of the LP980 spectrometer. The LP980 was also equipped with a cryostat, which allowed the acquisition of temperature-dependent PL spectra at room temperature (RT) and 77 K.
Figure 1 Edinburgh Instruments a) FS5 Spectrofluorometer, b) FLS Photoluminescence Spectrometer, and c) LP980 Transient Absorption Spectrometer.
Effect of DPA-BO Dopant Concentration with FS5
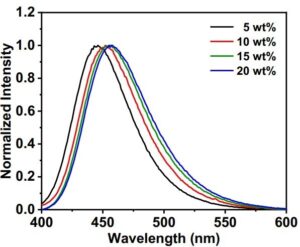
The compound’s PL spectra at different DPA-BO dopant concentrations doped in bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) thin films were investigated using the FS5 Spectrofluorometer. The DPA-BO concentration varied from 5-20 wt%. The PL spectra showed that as the dopant concentration increases, a shift towards longer wavelengths of the compound’s emission peak is observed (Figure 2). This behaviour can be attributed to the π–π stacking of the BO moiety. Further experimental techniques, such as time-of-flight secondary ion mass spectrometry, showed that the TADF compounds were not well-dispersed in their host matrix, confirming intermolecular solid interactions, which could potentially improve the charge-transfer (CT) processes.
Figure 2 Normalised PL spectra of the 5-20 wt% DPA-BO doped in DPEPO thin films at RT using an FS5 Spectrofluorometer. Figure reproduced from Nature Communications under CC BY 4.0.
Lifetime of the Prompt and Delayed Emission with FLS
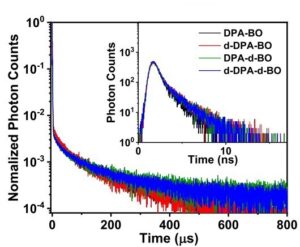
The prompt and delayed fluorescence of the 10 wt% doped films were measured using the FLS photoluminescence spectrometer. An EPL-375 pulsed laser diode emitting at 375 nm and operating in TCSPC mode was used as an excitation source. The decays were fitted by a biexponential model yielding around 3.5 ns for the prompt fluorescence (Figure 3 inset) and 59-67 μs for the delayed fluorescence (Figure 3).
Figure 3 Prompt (inset) and delayed fluorescence of all 10 wt% doped films, using an FLS-series spectrometer. Figure reproduced from Nature Communications under CC BY 4.0.
ΔES-T Splitting Measured with LP980
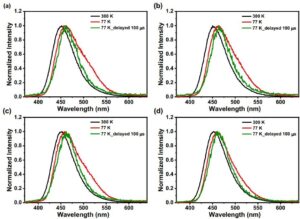
The LP980 was equipped with a liquid nitrogen cryostat, allowing the researchers to study the effect of temperature on the emission down to 77 K. Figures 4a-d show the temperature-dependent delayed and non-delayed PL spectra of the 10 wt% TADF compounds using the LP980’s iCCD camera. These measurements show the spectra of each TADF compound at RT and their fluorescence and phosphorescence (delayed by 100 μs) spectra at 77 K. From these spectra, the researchers could calculate the ΔE(S1-T1) and ΔE(S1-T2) splitting energies to be around 0.08 eV and 0.01 eV, respectively. These numbers suggest a possible reverse intersystem crossing (RISC) process from T2 states to S1.
Figure 4 Temperature-dependent PL spectra at RT and fluorescence and phosphorescence (delayed by 100 μs) spectra, using an LP980, an iCCD camera and a cryostat, of 10 wt% a) DPA-BO, b) d-DPA-BO, c) DPA-d-BO, d) d-DPA-d-BO. Figure reproduced from Nature Communications under CC BY 4.0.
Photostability Investigation with FS5
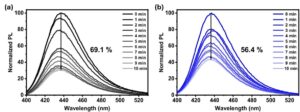
The researchers examined the PL stability of the TADF compounds doped in mCBP (1,3,5-tri(m-pyrid-3-yl-phenyl)benzene) at RT by continuously irradiating the samples with the 150 W Xenon lamp of the FS5. mCBP is an organic molecule commonly used as a host material in OLEDs. The measurements showed that after 10 minutes of CW illumination by the xenon lamp, the PL intensity of the compounds dropped. For instance, for the 10 wt% DPA-BO and d-DPA-d-BO, the PL intensity dropped by 69.1% and 56.4%, respectively (Figure 5a and 5b). These results indicate a positive deuteration effect on the photostability of the TADF emitters.
Figure 5 PL spectra of 10 wt% a) DPA-BO, and b) d-DPA-d-BO doped in mCBP over 10 minutes of CW xenon lamp irradiation. Figure reproduced from Nature Communications under CC BY 4.0.
Conclusion
The Edinburgh Instruments FS5 Spectrofluorometer, FLS Photoluminescence Spectrometer, and LP980 Transient Absorption Spectrometer were used to study the operational stability of TADF compounds for potential applications in OLED technology. Using the spectral and time-resolved spectral data, the researchers proposed innovative molecular engineering strategies at the subatomic level, such as targeted deuteration on donor and/or acceptor units, to enhance TADF OLED operational efficiencies.
Full Publication
The results presented in this Research Highlight were published in Nature Communications, and more details are available at https://doi.org/10.1038/s41467-023-42019-6.