Surfaced Enhanced Raman Scattering (SERS)
Introduction
Surfaced enhanced Raman scattering, or spectroscopy, (SERS), is an enhancement technique used to overcome the challenges seen with weak Raman scattering. SERS provides all the advantages of Raman spectroscopy whilst also offering higher sensitivity thorough scattering enhancement and fluorescence quenching. SERS can increase the Raman signal by up to 1010-15, allowing the analysis of single molecules by Raman spectroscopy.
Figure 1 highlights the enhancement that can be gained from using Surfaced enhanced Raman scattering (SERS). Spectra of 0.1 mM 4-nitrothiophenol are plotted with and without gold nanoparticles revealing the massive enhancement provided by the nanoparticles.
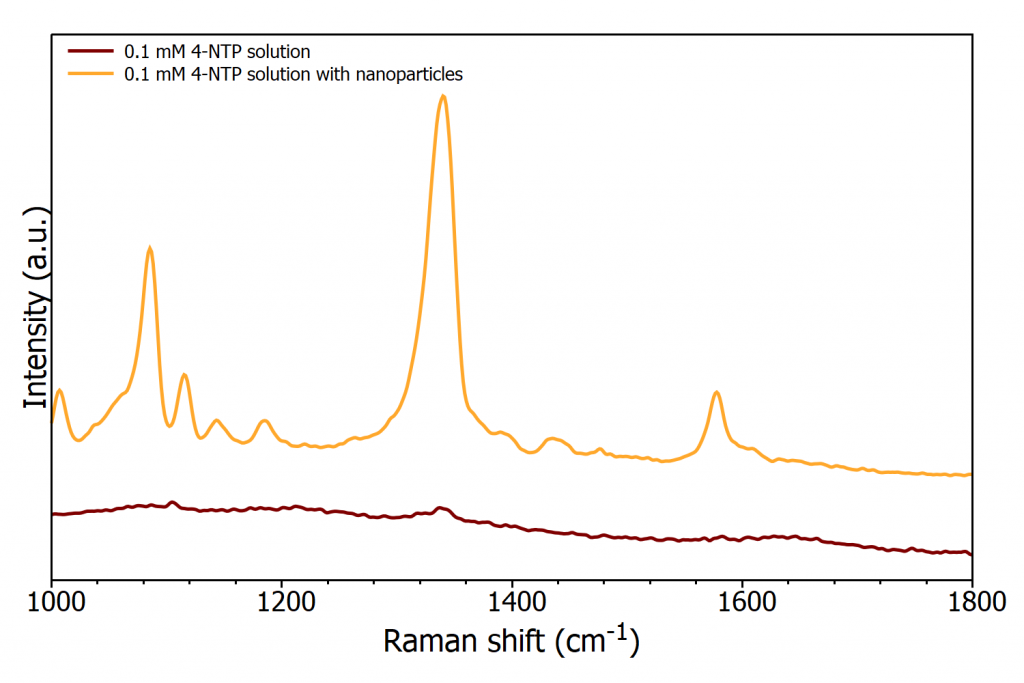 Figure 1: 0.1 mM solution of 4-NTP with and without gold nanoparticles measured on the RM5 Raman Microscope
Figure 1: 0.1 mM solution of 4-NTP with and without gold nanoparticles measured on the RM5 Raman Microscope
The SERS effect was first observed by Fleischmann, Hendra, and McQuillan at the University of Southampton in 1974. Enhancement was unexpectedly seen on the Raman spectra for pyridine adsorbed onto a roughened silver electrode.1 This led to the realisation that bringing molecules into proximity (i.e. adsorbing) with arrangements of nanoparticles, or metal surfaces that have nanoscale roughness, caused an enhancement of their Raman scattering intensity, disproportional to the concentration of molecules present. Two different groups confirmed these findings, each suggesting a different mechanism for the newly observed phenomena. Jeanmarie and van Duyne proposed the enhancement observed was due to electrochemical electric fields at the metal surface, this theory is commonly referred to as electromagnetic enhancement. 2 During the same period, Albretch and Creighton hypothesised the enhancement was due to the formation of a metal-molecule complex, termed chemical (or charge transfer) enhancement.3
Here the main area discussed will be the use of metals to provide enhancements, however there are some studies that show the potential of non-metallic materials. The SERS technique requires a roughened metal surface and the correct laser excitation, therefore no modifications of a Raman system are required. SERS offers all the standard advantages of Raman spectroscopy e.g. rapid analysis with minimal sample preparation plus increased Raman intensity and therefore sensitivity. The enhancement observed depends strongly on the optical properties of the SERS substrate, the characteristics of the laser excitation, the sample and its Raman cross-sections.
A SERS spectrum can differ quite significantly from a normal Raman spectrum due to metal-molecule interactions. The vibrational bands enhanced will be those closest to the metallic surface. This leads to non-reproducible spectra being acquired, this is one of the issues which has prevented SERS becoming a more commonly used tool in sample analysis. Additionally, to obtain reproducible spectra the nanostructures need to be uniform offering homogenous enhancement across the entire substrate. One way to overcome this issue is to functionalise the nanoparticles so that you purposefully enhance the area of the spectrum of interest.
The SERS Effect
The exact mechanisms of the SERS effect are still under debate today; however, it is largely accepted that both electromagnetic enhancement and chemical enhancement play a role, with electromagnetic effects having a more significant enhancement, Figure 2.4
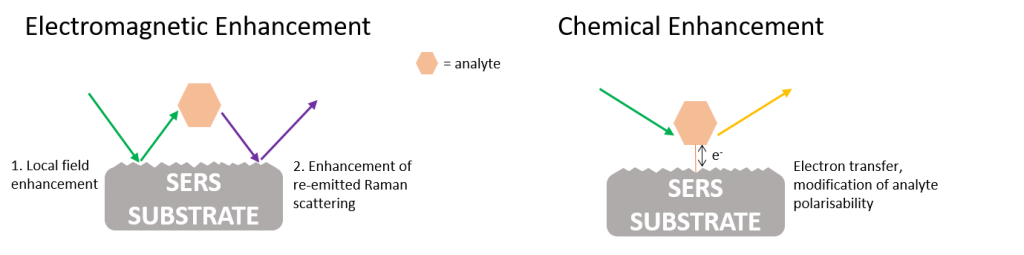 Figure 2: Electromagnetic enhancement and chemical enhancement mechanisms for SERS
Figure 2: Electromagnetic enhancement and chemical enhancement mechanisms for SERS
Electromagnetic Enhancement
Electromagnetic enhancement is the main contributor to the SERS effect, with enhancements of up to 1010 being attributed to it. Electromagnetic enhancement originates from two contributions; the local field enhancement, and the re-radiation enhancement. The effect arises after an analyte is adsorbed onto a roughened surface and excitation source causes a plasmon to be formed. A roughened surface is required to give a perpendicular component to the plasmon. The plasmon energy instigates the Raman process to occur in the analyte, the energy is transferred back into the plasmon, and the scattered radiation can be detected by the spectrometer. Hot spots give further enhancement, they are formed when two nanostructures are close enough to couple with each other. When a molecule resides in the hot spot the electromagnetic field they experience is increased, providing further enhancement.
Electromagnetic enhancement is independent of the type of molecule and reliant on the substrate and its roughness. The molecule needs to be placed about 1-10 nm from the substrate, meaning in comparison to the chemical enhancement theory, it is a long-range effect.
Chemical Enhancement
Chemical enhancement has a much lesser effect on the Raman enhancement, typically 102-104. Enhancement arises due to the interaction of the molecule with the substrate causing a modification of the polarisability of a molecule and thus the Raman cross-section of vibrational modes. Additionally, charge transfer can occur where there is an exchange of electrons between the Fermi level of the metal and the lowest unoccupied molecular orbital (LUMO) or highest occupied molecular orbital (HOMO) of the molecule. Separation between sample and substrate for the enhancement to occur is in the order of angstroms, making it a short-range effect.
SERS Substrates
Any material can serve as a SERS substrate if it can support plasmon activity at the excitation wavelength. Several factors affect the SERS enhancement at the substrate level. The ideal substrate should have high SERS activity, this involves optimising the size, shape, and metal roughness for maximum enhancement. The analyte must adsorb onto the surface efficiently and have a higher Raman cross-section than any potential interferants within the sample. If an interferent has a higher Raman cross-section it will dominate the spectrum. The substrate should be uniform, providing the same enhancement over the entire substrate whilst also being clean and having high stability for a long shelf life. Finally, SERS substrates should be simple to produce at low cost. However, it is difficult to achieve this list and in reality, trade-offs need to be made to suit individual applications. For example, if you are interested in trace detection the enhancement factor is the most critical variant, whereas if you are interested in quantitative analysis reproducibility is critical between SERS measurements.
The metal chosen for SERS enhancement is crucial to the amount of enhancement seen. Gold and silver are the most commonly used metals for SERS. This is due to their chemical stability as well as their plasmon resonance frequencies fall within the visible and NIR range, which are the most commonly used excitation wavelengths in Raman spectroscopy. The most frequent metal of choice is gold due to its higher chemical stability and lower toxicity as silver is prone to oxidation and reacting with sulfur compounds in the atmosphere. However, gold absorbs strongly at 500 nm and for excitation with the popular 532 nm laser, silver nanoparticles are favoured. Other transition metals have been tested for their enhancement effects, such as platinum and iron, however their reported enhancement levels are substantially lower than those seen for gold, silver, or copper. They do however, show greater enhancement when used in the UV range.
The most common types of SERS substrates are nanoparticles in solution, nanoparticles immobilised on solid substrates, and nanostructures fabricated onto solid substrates. By altering the shape of the nanoparticles more enhancement can be observed, this is due to the presence of hot spots at edges and corners. Common shapes used for high enhancement include nanorods, nanocubes, and nanostars, Figure 3. These shapes provide hot spot regions, indicated in dark green, which provide increased enhancement.
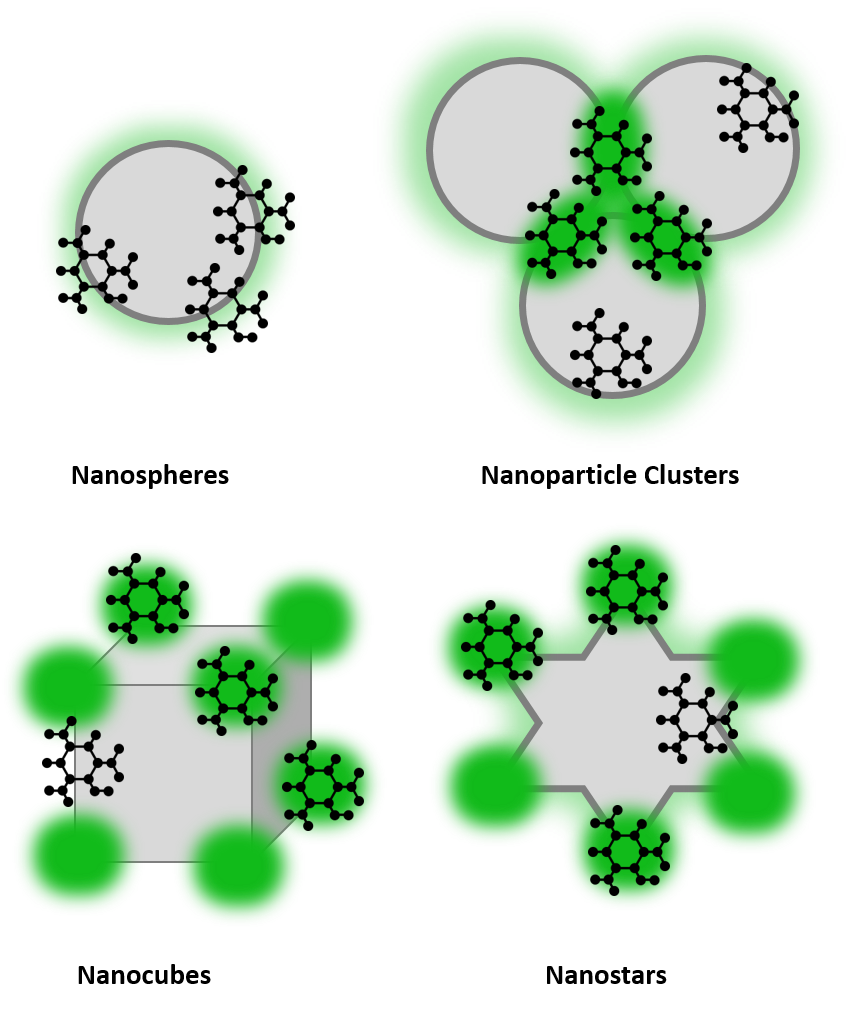 Figure 3: SERS substrate examples
Figure 3: SERS substrate examples
SERRS
Surface enhanced resonance Raman scattering (SERRS) delivers further enhancement by incorporating resonance Raman spectroscopy. Resonance Raman has been previously discussed in our blog article. To summarise, in resonance Raman spectroscopy the laser excitation source is chosen to be close to the frequency of an electronic transition in the sample. Resonance Raman spectroscopy can provide enhancements in the range of 102-106 and as a technique is much more understood than SERS.
If using an analyte with a resonant chromophore and a metallic nanoparticle SERRS is possible. The enhancement from SERRS is greater than that of SERS and resonance Raman individually, comparable to or exceeding the sensitivity of fluorescence. The extra enhancement seen is a chemical enhancement effect based on charge transfer resonance.
SERRS overcomes some of the issues of its two components. The problem of fluorescence seen with resonance Raman is removed in SERRS as the nanoparticles quench this fluorescence. With the combination of the two enhancement techniques you gain both vibrational and electronic information. However, the presence of a metal surface can alter where the resonance lies and the metal can also cause changes in the analyte, for example protein conformation changes. If these issues can be resolved for the sample SERRS is a good labelling technique due to its large enhancements, quenched fluorescence, and specific enhancement of the chromophore.
Conclusion
Overall SERS and SERRS are two powerful Raman techniques overcoming the shortfalls of weak Raman scatter under standard conditions for both quantitative and qualitative analysis. Enhancement allows for lower laser powers to be used, protecting the sample, and a shorter integration times, speeding up the acquisition. Whilst there are still issues with fully understanding the mechanisms behind the enhancements and with the reproducibility and stability of substrates, SERS/SERRS offers real potential to gain more information from analytes at extremely low concentrations. The applications of SERS/SERRS can be seen across various fields, for example: SERS probes for in-vitro studies, SERS immunoassays, single molecule detection, materials analysis, and DNA detection. SERS probes can be used to monitor experiments or simply to track levels of a specific analyte, for example monitoring glucose concentrations which is explored in a recent application note.
References
1. Fleischmann, M., Hendra, P. J. & McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. (1974) doi:10.1016/0009-2614(74)85388-1.
2. Jeanmaire, D. L. & Van Duyne, R. P. Surface raman spectroelectrochemistry. Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. (1977) doi:10.1016/S0022-0728(77)80224-6.
3. Albrecht, M. G. & Creighton, J. A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. (1977) doi:10.1021/ja00457a071.
4. Langer, J. et al. Present and future of surface-enhanced Raman scattering. ACS Nano 14, 28–117 (2020).
The RM5 Raman Microscope
If you are working in the field of Raman Spectroscopy, our Raman Confocal Microscope may be the perfect partner for your Raman research. For further information please Contact Us.








