Our FLS980 Spectrometer
The FLS980 has now been discontinued and is superseded by our new and improved FLS1000 photoluminescence spectrometer.
The FLS1000 is a state of the art spectrometer for the most demanding fluorescence analysis applications in Photophysics, Photochemistry, Materials Sciences and Life Sciences.
Discover the FLS1000 photoluminescence spectrometer.
Fluorescence Analysis
Why not view our complete range of fluorescence analysis instrumentation and find the best instrument for your fluorescence research. For further information on any of our products, please contact us.
Product Description
FLS980 Spectrometer Upgrades
We offer a full range of spectrometer upgrades for the FLS980 including configuration, light source and software upgrades in addition to monochromator options, detector option and fluorescence and phosphorescence upgrades. View the full range of FLS980 upgrades.
For further information regarding the FLS980, please contact a member of our sales team who will be delighted to help.
The FLS980 combines ultimate sensitivity with high spectral resolution and excellent stray light rejection, setting the industry the standard for both steady state and time-resolved photoluminescence spectroscopy in the ultraviolet and near-infrared spectral range. The modular construction enables the system to be readily configured to meet your individual research needs as well as be reconfigured as your research changes.
The FLS980 is supplied with computer-controlled, triple grating turret monochromators, with up to three different gratings permanently fitted. This allows a large spectral range to be covered while providing the maximum ease of use.
Two detectors with independent exit slits can be mounted on a single emission monochromator (three on double emission monochromator). Single photon counting PMTs are available to cover the wavelength range from 200 nm – 1700 nm, while analog detectors extend the wavelength range to beyond 5000 nm.
A signal-to-noise ratio of >25,000:1 for the Water Raman Spectrum under standard measurement conditions is guaranteed. This sensitivity allows the spectra of weak dye solutions (< 100 fM) to be routinely measured.
An optional upgrade to the sensitivty of the FLS980 is available. This takes the signal-to-noise ratio to >35,000:1 guaranteed.
The FLS980 has a USB interface and all modes of operation are controlled by ONE data acquisition module and ONE all-inclusive F980 software package for data acquisition and analysis. Light source, detector, grating, slits, polarizers are all computer-controlled for accurate and precise measurements.
Excitation Sources
The FLS980 comes standard with a 450 W ozone free xenon arc lamp that covers a range of 230 nm to 1000 nm for steady state measurements. A variety of other sources can be integrated including microsecond flashlamps, nanosecond flashlamp, pulsed diode lasers (EPL Series), pulsed light emitting diodes (EPLED Series), supercontinuum lasers, Ti:sapphire lasers, Q-switched solid state lasers and OPOs, dye lasers, and infrared CW and pulsed lasers for upconversion measurements.
Monochromators
Single and double grating Czerny-Turner monochromators are available in the FLS980 with 300 mm (or 2 x 300 mm) focal length, high optical throughput, excellent stray light rejection and low temporal dispersion. The monochromators feature triple grating turrets with up to three gratings on each turret and computer-controlled slits.
Detectors
A full range of detector options are available to enhance the range of spectral coverage and or to reduce the instrumental response width for lifetime measurements. The instrument comes standard with a R928P PMT detector in a cooled housing which covers a range from 200 nm – 870 nm, in TCSPC mode the instrumental response width is approximately 600 ps. Optional detectors include: high speed PMTs in a cooled housing with instrument response <100 ps, MCP-PMT in cooled housings with a response <25 ps, NIR-PMTs to cover spectral ranges out to 1700 nm with photon counting sensitivity and speed, InGaAs detectors with spectral coverage up to ~1.65 μm, 2.05 um and 2.55 μm, InAs and InSb detectors to cover out to 5.5 μm.
Sample Holders
At the heart of the FLS980 is an exceptionally large sample chamber that allows access to the sample from all sides, top and bottom. This ensures compatibility and simplifies access to a variety of sample holders.
Fluorescence Analysis
For further information on our range of fluorescence analysis instrumentation, why not visit our fluorescence products overview.
Technical Specifications
| Specifications | FLS980 Spectral |
FLS980 Fluorescence Lifetime |
FLS980 Phosphorescence Lifetime |
|---|---|---|---|
| System | A computer-controlled spectrometer for measuring steady state luminescence spectra in the UV-NIR spectral range | Computer-controlled spectrometer for measuring fluorescence lifetimes spanning the range from picoseconds to microseconds | Computer-controlled spectrometer for measuring phosphorescence lifetimes spanning the range from microseconds to seconds |
| Mode of operation | Single Photon Counting – Steady State Measurements | Time-Correlated Single Photon Counting (TCSPC) | Time-Resolved Single Photon Counting – Multi-Channel Scaling (MCS) |
| Lifetime range | Milliseconds to hours | 100 ps – 50 µs (nF920) 5 ps – 50 µs (fs laser/MCP-PMT) |
1 µs – 10 s (µF2) 10 ns – 10 s (pulsed laser) |
| Sensitivity | >25,000:1 | n/a | n/a |
| Excitation Sources | |||
| Type | 450 W ozone-free xenon arc lamp | Nanosecond flashlamp (nF920) | Microsecond flashlamp (µF2) |
| Mode of operation | Steady state | TCSPC | MCS |
| Spectral range | 230 nm – >1000 nm | 200 nm – >400 nm | 200 nm – >1000 nm |
| Pulse width | n/a | <1 ns | 1-2 µs |
| Options | Ozone generating lamp with spectral range 200 nm – >1000 nm |
Picosecond pulsed diode lasers (EPL Series) and pulsed LEDs (EPLED Series) | Low to medium repetition rate pulsed lasers |
| Monochromator Specifications | |
|---|---|
| Type | Czerny-Turner with Triple Grating Turret |
| Focal length | 300 mm, (double monochromators: 2 x 300 mm) |
| Slits | <10 µm to 12 mm fully computer-controlled |
| Stray light rejection | 1:10-5 (single monochromators), 1:10-10 (double monochromators) |
| Gratings | Mounted to triple grating turret, fully computer controlled, contact us for more information |
| Photomultiplier Options | |||||
|---|---|---|---|---|---|
| Photomultiplier | R928P | R2658P | NIR PMT | High Speed | MCP-PMT |
| Spectral range | 200 nm – 870 nm |
300 nm – 1010 nm |
300 nm – 1700 nm |
230 nm – 870 nm |
200 nm – 850 nm |
| Dark count rate | <50 cps (-20°C) |
<100 cps (-20°C) |
<200 kcps (-80°C) |
<100 cps (0°C) |
<50 cps (-20°C) |
| Response width | 600 ps | 600 ps | 800 ps | 200 ps | <50 ps |
A wide variety of photomultipliers and analogue detectors are available.
Please visit the FLS980 Upgrades page for more information or contact us directly.
Software
F980 spectrometer operating software is at the heart of all our fluroescence spectrometers and is a fully comprehensive, user-friendly data analysis software package. Irrespective of system configurations, this software provides the user with complete control.
The F980 software is Windows 7, 8 and 10 compatible and is based on a data centred design that enables the user to focus on their measurement. This guarantees ease-of-use in the operation of a modular and potentially complex spectrometer.
Measurement set-up and data acquisition is made through an intuitive menu system. Key spectroscopic parameters are easily accessed through functional groupings, while common measurement routines can be saved as method files to allow previous experiments to be easily repeated. Tabbed dialogue boxes and particular scan parameters are always visible during set-up. The current status of the instrument is also continuously displayed.
A unique feature of the F980 software is that all modes of data acquisition, including spectral scanning and lifetime acquisition in both MCS and TCSPC modes, are controlled from within one software package. Modern light sources, detectors, complex sample holders (plate reader, XY sample stages, titrator) and cooler options (thermostated sample holders and cryostats) are supported and fully software controlled.
Upgrade to FAST (Fluorescence Analysis Software Technology) software for the advanced analysis of fluorescence and phosphorescence decay kinetics.
Measurement Examples (Steady State)
Excitation and Emission Scans
Excitation and emission spectra are standard measurements in fluorescence spectroscopy. The figure demonstrates a measurement of a well documented standard test solution of anthracene in degassed cyclohexane.
Sample: Anthracene in cyclohexane (10-5M). Measurement conditions: λex = 358 nm for emission scan, λem = 400 nm for corrected excitation scan, Δλex = Δλem = 0.4 nm, step size = 1 nm, integration time = 1 s.
Synchronous Scans
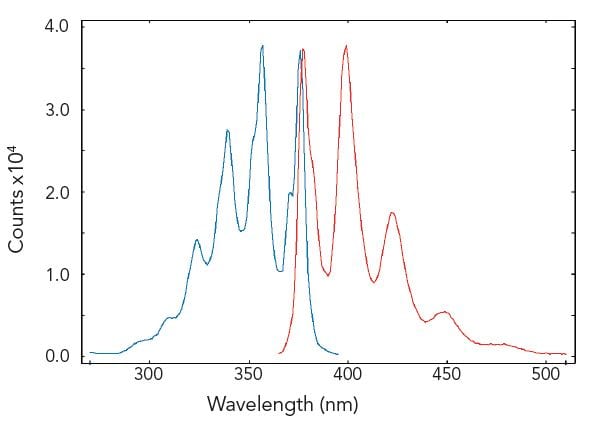
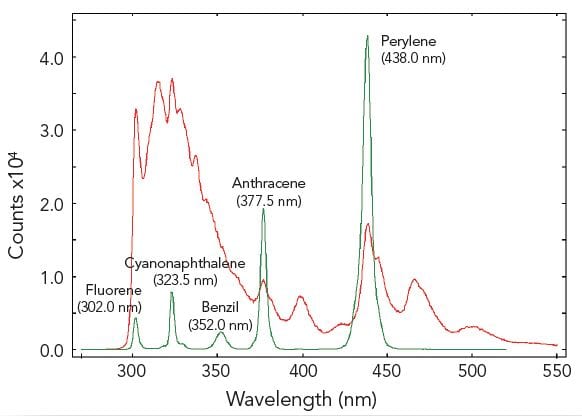
In synchronous scans, both excitation and emission monochromators are scanned synchronously with a pre-set offset. The figure demonstrates a sample of five different aromatic hydrocarbons dissolved in cyclohexane, measured with a conventional emission scan (red) and a synchronous scan with zero offset (green). The five hydrocarbons are resolved by the synchronous scan.
Sample: Five aromatic hydrocarbons dissolved in cyclohexane. Measurement conditions: λex = 280 nm for emission scan, Δλex = Δλem = 0.5 nm, step size = 0.5 nm, integration time = 1 s, offset = 0 nm.
Kinetic Scans
Kinetic scans reveal temporal changes of the sample fluorescence at fixed excitation and emission wavelengths. Luminescence emission in the milliseconds to seconds range, such as long phosphorescence, chemical reactions or chemical migration in cells, can be studied. As an example, using the FLS980 in T-geometry for dual wavelength detection, simultaneous measurements of the Ca2+ active fluorophore Indo-1 can be made with both emission arms set to different wavelengths.
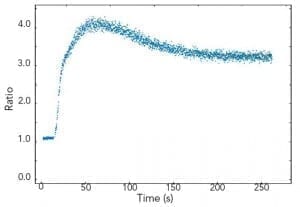
Sample: Human platelets cells loaded with Indo-1 in 1 mM Ca2+. Measurement conditions: λex = 340 nm, λem1 = 485 nm, λem2 = 410 nm, Δλex = Δλem = 1 nm, integration time = 0.5 s.
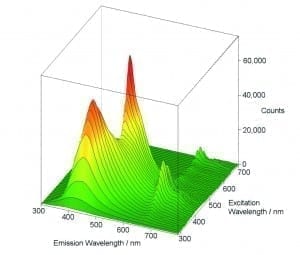
Excitation – Emission Maps (EEM)
The variety of measurement, display and analysis options allows easy and fast investigation of unknown luminescent samples or samples which contain different fluorophores. One method is to measure a series of emission scans within a selected range of excitation. The result is then demonstrated either in a 3D plot or in a contour plot.
Sample: Three organic dyes in solution: naphtalene, anthracene perylene. Measurement conditions: Xe1, R928P, 280 nm ≤ λex ≤ 460 nm, 310 nm ≤ λem ≤ 620 nm, Δλex = Δλem = 2 nm, integration time = 0.5 s, repeats per scan = 1.
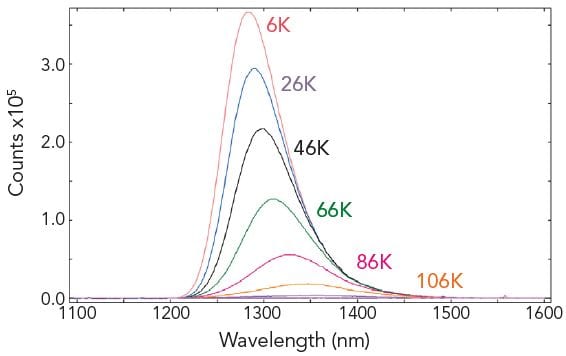
Temperature Maps
The F980 software can communicate with Oxford Instruments Optistat DN (liquid nitrogen) and Optistat CF (liquid helium) cryostats (along with TE controlled sample holders). Temperature maps can be made by acquiring a series of emission, excitation or synchronous scans for a predefined temperature range. The individual measurements are automatically started when the target temperatures are reached.
Sample: CuInSe2 (a material used for photovoltaic cells). Measurement conditions: F980 controlled Optistat, Xe1, NIR-PMT, λex = 694 nm, Δλex = 10 nm, Δλem = 5 nm, step size = 1 nm, integration time = 0.2 s. Temperature range: 6 K – 106 K, step 20 K.
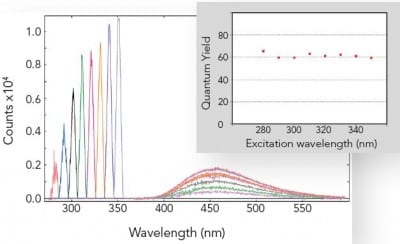
Absolute Quantum Yield Measurements
The absolute method for fluorescence quantum yield measurements is becoming more widely used than the relative method, as it does not require a quantum yield standard. This is readily applicable to liquids, films and powders and can be extended into the near infrared spectral range.
The picture shows the independence of the fluorescence quantum yield from the wavelength of excitation for a standard organic dye. The graph shows the area of absorption for eight different excitation wavelengths on the left, while on the right it shows the corresponding emission spectra, scaled by a factor of 5. The inset shows the calculated quantum yields.
Sample: Quinine bisulphate in perchloric acid. Measurement conditions: integrating sphere, Δλex = 5.0 nm, Δλem = 0.5 nm, integration time = 0.3 s.
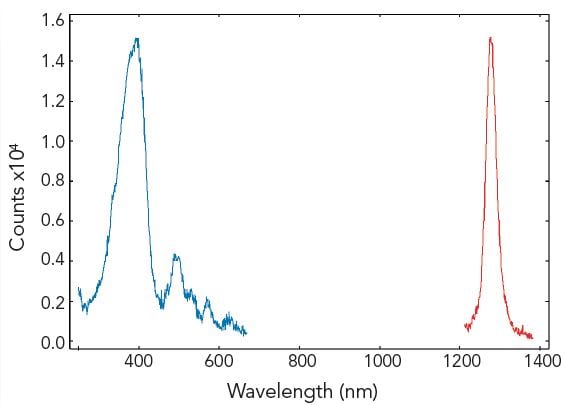
Singlet Oxygen Emission
The emission of singlet oxygen is known to be very weak and, historically, powerful laser excitation has been used to monitor this. However, both excitation and emission spectra of singlet oxygen can be measured using the FLS980 with a broadband xenon lamp. The figure demonstrates a measurement of singlet oxygen luminescence generated from hematoporphyrin in ethanol. In a mixture of photosensitizers, the excitation spectrum may be used to identify the singlet oxygen generator.
Sample: 10-5 M hematoporphyrin in ethanol. Measurement conditions: λex = 380 nm for emission scan, λem = 1270 nm for excitation scan, Δλex = Δλem = 2.0 nm, step size = 1.0 nm, integration time = 3.0 s.
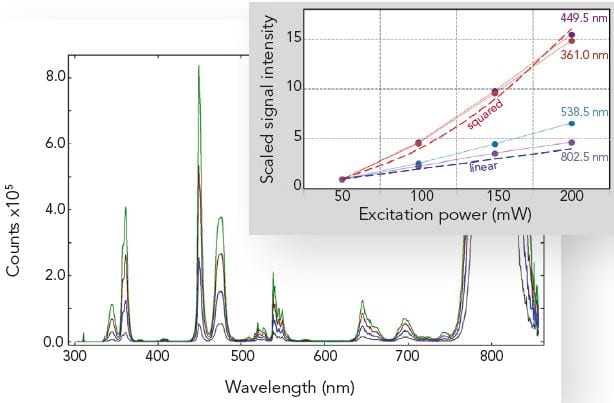
Fluorescence Upconversion
Fluorescence upconversion materials absorb light in the near infrared spectral range and produce emission of shorter wavelengths in the visible spectral range. These upconversion materials are currently the focus of research for their use in dye sensitized solar cells (DSSC). Efficient upconversion is important for the efficiency of solar panels in the near infrared part of the sun’s spectrum.
The picture below shows the upconversion emission of an erbium-ytterbium doped TiO2 powder, for four different levels of excitation power at 980 nm. As shown in the insert, some of the upconversion lines scale close to linear with excitation power, whereas others scale approximately to the square of the excitation power.
Samples: Er3+/Yb3+ doped TiO2. Measurement conditions: 1 W diode laser with power adjustment @ 980 nm, R928P, Δλem = 0.25 nm, integration time = 0.5 s.
Other steady state measurement examples: Steady state fluorescence anisotropy, contour plots, water quality assessments, excimer equilibrium, reflection, absorption and quantum yield measurements of phosphor powders, chromaticity and much more.
Measurement Examples (Time-Resolved – TCSPC)
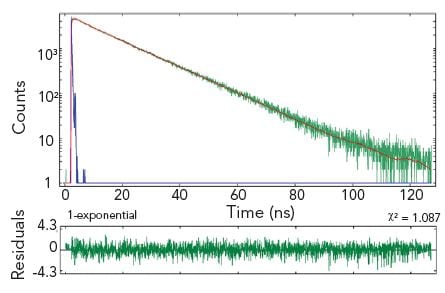
Single and Multiple Exponential Decays
Fluorescence lifetime is an excellent parameter for analysing the interaction of fluorophores with their micro-environment, such as solvents, neighbouring fluorophores or non-fluorescing molecules. These “environmental” effects will reduce the natural decay process (characterised by the natural lifetime), to shorter and often more complex decay kinetics.
Most fluorescence decay kinetics are analysed by single or multiple exponential models. The user fits the raw data to a specific model. The quality of the fit will determine whether or not the selected model was appropriate, and – if it was – the result will provide the fit parameter such as lifetimes and pre-exponential factors.
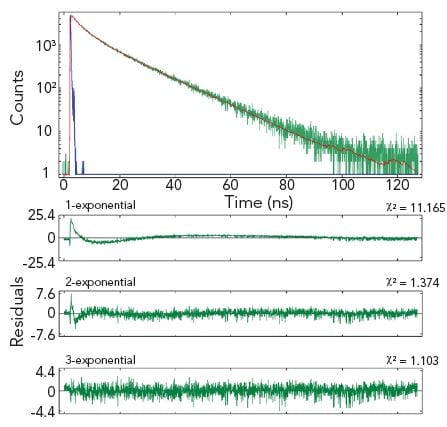
The example shows two measurement results of the same homogeneous solution, taken at two different emission wavelengths. The decay at the shorter wavelength is clearly a single exponential, the decay at the longer wavelength is best characterised by three exponential components.
Sample: Hematoporphyrin IX in phosphate buffer (pH 7.2)
Measurement conditions: EPL 405, MCP-PMT, λex = 398 nm, Δλem = 1.0 nm, rep rate = 1 MHz, λem = 620 nm (left and right graph)
Data analysis: Multi-exponential reconvolution, confidence intervals verified by support plane analysis (FAST). τ1 = 15.02 ± 0.03 ns (left). τ2 = 14.80 ± 0.20 ns, τ2 = 4.62 ± 0.55 ns, τ3 = 0.81 ± 0.20 ns (right).
Time-Resolved Fluorescence Anisotropy
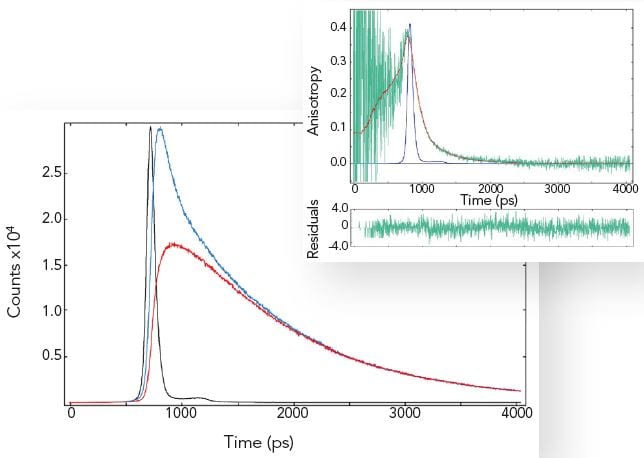
By exciting the sample with vertically polarised light and recording the emission in both the vertical and horizontal plane, one can calculate the fluorescence anisotropy of a homogeneous sample. The fluorescence anisotropy reveals the average rotational diffusion time of the molecules.
The measurement example shows that rotational diffusion in the picosecond time scale can be accurately measured. Most samples show rotational diffusion. To avoid this effect when precise fluorescence lifetime measurements are required, the emission polariser must be set to magic angle conditions (and vertically polarised excitation used).
Sample: POPOP in cyclohexane (IRF, decays with parallel and crossed polariser – left plot), fluorescence anisotropy (raw data and fit – right plot). Measurement conditions: EPL 375, MCP-PMT, λex = 375 nm, Δλex = 2.0 nm, λem = 390 nm, Δλem = 2.0 nm.
Data analysis: Full anisotropy reconvolution (FAST) with ellipsoidal rotor model. The rotation diffusion times are 110 ps, 150 ps and 620 ps respectively. A spherical rotor model results in a fit with significantly increased chi-square. POPOP is a rod like molecule.
Other TCSPC measurement examples: Time-resolved emission spectroscopy (TRES), monomer-excimer kinetics, solvent relaxation dynamics and much more.
Measurement Examples (Time-Resolved – MCS)
Time-Resolved Measurements of Lanthanides
The photoluminescence emission lifetime of lanthanides extends over a large time range from nanoseconds to seconds where the method of choice for time-resolved measurements is the MCS technique. Due to the high dynamic range and the accuracy resulting from counting statistics, complex decay analysis can be performed.
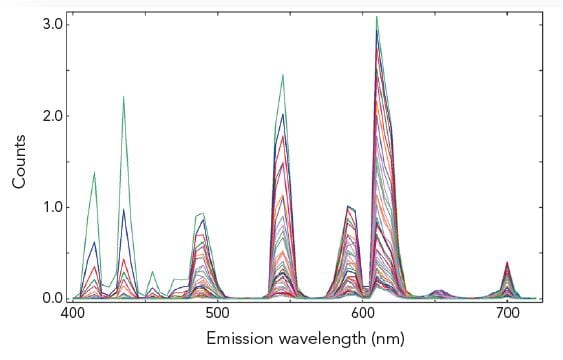
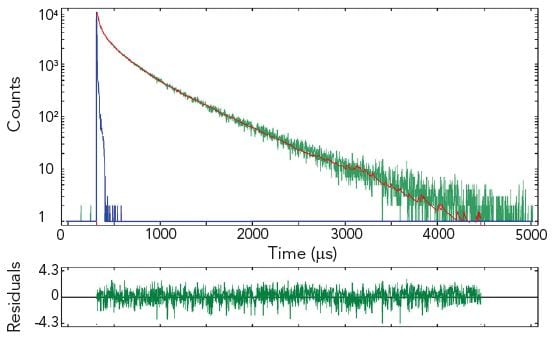
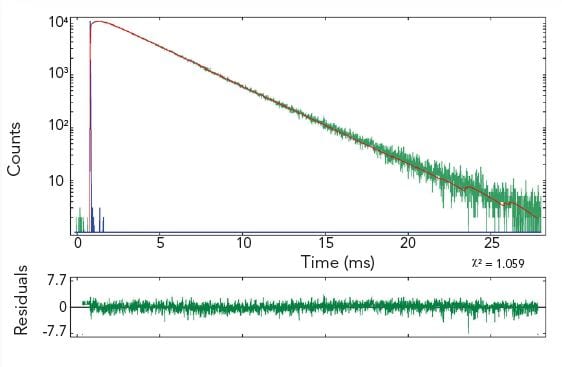
The pictures show time resolved measurements from a lanthanide doped glass sample at two different emission wavelengths. At the shorter wavelength the decay is best fitted with a three exponential terms, while at the longer emission wavelength the initial rise is followed by a millisecond decay.
Sample: Rare-earthed doped glass
Measurement conditions: μF2, λex = 370 nm, Δλex = Δλem = 2.5 nm, rep rate 100 Hz, step size = 10 nm, spectra produced for every 50 μs (Top Left). μF2, λex = 370 nm, λem = 430 nm, Δλex = Δλem = 2.5 nm, rep rate 100 Hz, step size = 10 nm, measurement time = 2 min (Top Right). μF2, λex = 370 nm, λem = 612 nm, Δλex = Δλem = 1.7 nm, rep rate 20 Hz, measurement time = 8 min (Bottom Left).
Data Analysis: Multi-exponential reconvoltution. Good fit results were achieved with four exponential decay model (Top Right) and model comprising two exponential rise and one decay function (Bottom Left).
Other MCS measurement examples: Time-resolved singlet oxygen measurements, time-resolved FRET measurements and much more.