Transient Absorption
Transient Absorption / Laser Flash Photolysis is a technique for studying transient absorption of chemical and biological species generated by a short intense light pulse from a pulsed laser source (‘pump source’).
This intense light pulse creates short lived photo-excited intermediates such as excited states, radicals and ions. All these intermediates are generated in concentrations large enough for chemical and physical interaction to occur and for direct observation of the associated temporally changing absorption characteristics. These absorption changes are recorded using a spectrally continuous Xenon lamp (‘probe source’) forming the background in a single beam absorption spectrometer. The probe source is operated in a pulsed mode to enhance the photon flux for measurements in short time ranges.
For further information or to discuss your requirements, please contact us.
Description
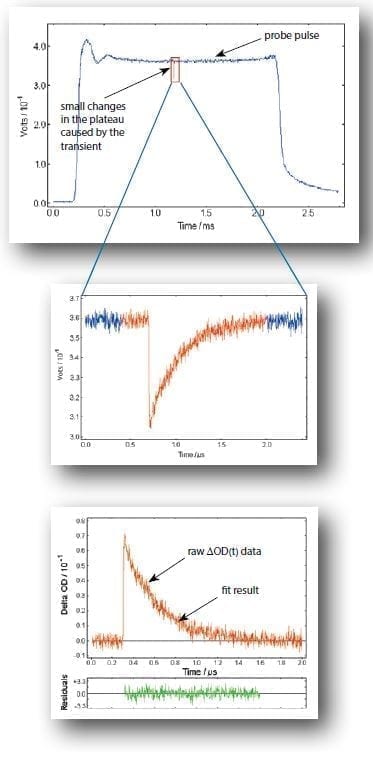 The sample being investigated is exposed to an intense laser pump pulse, which creates the transient species, and the probe source, which forms the background for the time dependant absorption measurement. For time scales in the microsecond and nanosecond range the required high background level of the probe light is created by the intense flash from the pulsed xenon lamp which – after some stabilisation period – reaches a sufficiently flat plateau.
The sample being investigated is exposed to an intense laser pump pulse, which creates the transient species, and the probe source, which forms the background for the time dependant absorption measurement. For time scales in the microsecond and nanosecond range the required high background level of the probe light is created by the intense flash from the pulsed xenon lamp which – after some stabilisation period – reaches a sufficiently flat plateau.
This plateau level represents the pre-photolysis background level of the transmitted light through the sample. At a pre-set time after lamp triggering, when the pulse plateau is flat, the excitation laser is triggered creating the transient species under investigation. The absorption of the transient species is usually time dependent and produces a time dependent change in the transmission of the sample.
The change in optical density, ΔOD, can then be analysed using exponential least squares fitting algorithms, resulting in transient lifetimes or rate constants.
To protect the sample against photobleaching by unnecessary radiation exposure between measurements and as a means to control background measurements, high speed shutters are operated to control the probe and laser beam prior to entering the sample. For laser induced emission measurements the probe shutter remains permanently closed.
Products
Applications
Transient absorption measurements / laser flash photolysis is applicable to liquid, gaseous, and solid samples.
Liquids are usually measured in a cuvette with the pump beam and the probe beam overlapping orthogonally (transverse excitation). In gaseous samples the concentration of the participating molecules is much lower and a co-linear setup between the pump and probe beam is preferred to improve the signal to noise ratio. Film samples, powders and non transparent bulk samples are generally studied in a diffuse reflectance setup.
- Transient Absorption and Photobleaching
- Triplet-Triplet Annihilation
- Oxygen Quenching of Transient Absorption Decays
- Spectrally Dependent Transient Kinetics
Publications
| Author | Year | Title | Journal | Additional details | Volume | Pages | Instruments | Publication details |
|---|---|---|---|---|---|---|---|---|
| Cédric Mongin et al. | 2016 | Direct observation of triplet energy transfer from semiconductor nanocrystals | Science Volume:351 Pages:369-372 Year:2016 | Journal:Science Volume:351 Pages:369-372 Year:2016 Instruments | 351 | 369-372 | Direct observation of triplet energy transfer from semiconductor nanocrystals Author:Cédric Mongin et al.Further info Journal:Science Volume:351 Pages:369-372 Year:2016 Instruments | |
| Zongle Li et al. | 2016 | An Ethylenediamine-Modified Graphene Oxide Covalently Functionalized with Tetracarboxylic Zn(II) Phthalocyanine Hybrid for Enhanced Nonlinear Optical Properties | Photochemical & Photobiological Sciences Volume:15 Pages:910-919 Year:2016 | Journal:Photochemical & Photobiological Sciences Volume:15 Pages:910-919 Year:2016 Instruments | 15 | 910-919 | An Ethylenediamine-Modified Graphene Oxide Covalently Functionalized with Tetracarboxylic Zn(II) Phthalocyanine Hybrid for Enhanced Nonlinear Optical Properties Author:Zongle Li et al.Further info Journal:Photochemical & Photobiological Sciences Volume:15 Pages:910-919 Year:2016 Instruments | |
| Leila Alibabaeia, et al. | 2013 | Solar water splitting in a molecular photoelectrochemical cell | PNAS Volume:110 Pages:20008-20013 Year:2013 | Journal:PNAS Volume:110 Pages:20008-20013 Year:2013 Instruments | 110 | 20008-20013 | Solar water splitting in a molecular photoelectrochemical cell Author:Leila Alibabaeia, et al.Further info | |
| Jamie C. Wang et al. | 2015 | Modulating Electron Transfer Dynamics at Dye–Semiconductor Interfaces via Self-Assembled Bilayers | The Journal of Physical Chemistry C Volume:119(7) Pages:3502-3508 Year:2015 | Journal:The Journal of Physical Chemistry C Volume:119(7) Pages:3502-3508 Year:2015 Instruments | 119(7) | 3502-3508 | Modulating Electron Transfer Dynamics at Dye–Semiconductor Interfaces via Self-Assembled Bilayers Author:Jamie C. Wang et al.Further info Journal:The Journal of Physical Chemistry C Volume:119(7) Pages:3502-3508 Year:2015 Instruments | |
| Dariusz M. Niedzwiedzki et al. | 2014 | Excited state properties of chlorophyll f in organic solvents at ambient and cryogenic temperatures | Photosynthesis Research Volume:121 Pages:25-34 Year:2014 | Journal:Photosynthesis Research Volume:121 Pages:25-34 Year:2014 Instruments | 121 | 25-34 | Excited state properties of chlorophyll f in organic solvents at ambient and cryogenic temperatures Author:Dariusz M. Niedzwiedzki et al.Further info | |
| Lesley Tilleman, et al. | 2015 | A Globin Domain in a Neuronal Transmembrane Receptor of Caenorhabditis elegans and Ascaris suum: Molecular Modeling and Functional Properties | The Journal of Biological Chemistry Volume:290 Pages:10336-10352 Year:2015 | Journal:The Journal of Biological Chemistry Volume:290 Pages:10336-10352 Year:2015 Instruments | 290 | 10336-10352 | A Globin Domain in a Neuronal Transmembrane Receptor of Caenorhabditis elegans and Ascaris suum: Molecular Modeling and Functional Properties Author:Lesley Tilleman, et al.Further info Journal:The Journal of Biological Chemistry Volume:290 Pages:10336-10352 Year:2015 Instruments |








